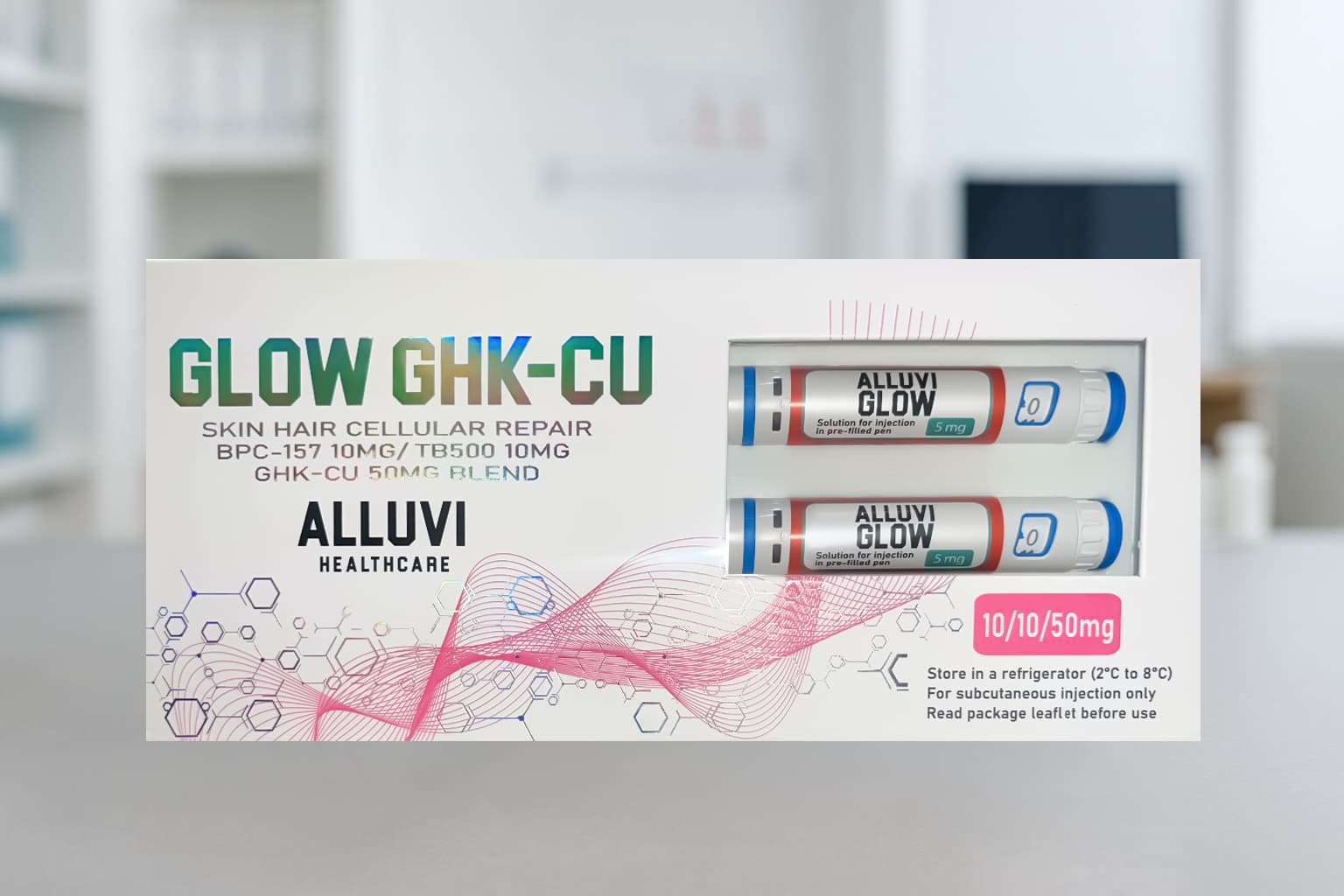
Glow 70mg GHK-CU – Premium Research-Grade Copper Peptide
⚠️ IMPORTANT REGULATORY NOTICE
READ BEFORE PROCEEDING:
- Cosmetic Use ONLY: GHK-Cu (Copper Tripeptide-1) is legally approved for cosmetic use in topical products.
- Injectable Form PROHIBITED: As of September 2023, FDA added injectable GHK-Cu to Category 2, prohibiting commercial compounding for injection.
- Research Use Declaration: This product is sold exclusively for in vitro laboratory research purposes.
- Not FDA-Approved Drug: GHK-Cu is not FDA-approved for treatment, cure, or prevention of any disease.
- NOT for Human Consumption: Not intended for human injection, ingestion, or therapeutic use.
Introduction to Glow 70mg GHK-CU Copper Peptide
Glow 70mg GHK-CU represents a premium research-grade copper peptide complex designed for qualified laboratories conducting investigations into skin regeneration mechanisms, collagen synthesis pathways, and gene expression modulation. As one of the most extensively studied peptides in regenerative medicine research, GHK-Cu (glycyl-L-histidyl-L-lysine copper complex) offers researchers a powerful tool for examining tissue remodeling, antioxidant defense systems, and copper-mediated biological processes.
Manufactured under pharmaceutical-grade conditions, our Glow 70mg GHK-CU formulation provides researchers with high-purity material suitable for in vitro studies, gene expression analysis, and mechanistic investigations—exclusively for laboratory research purposes.
Why “Glow” GHK-Cu?
The “Glow” designation reflects GHK-Cu’s extensive research applications in dermatological and cosmetic science:
- Decades of published research on skin regeneration mechanisms
- Wide use in cosmetic formulations (INCI: Copper Tripeptide-1)
- Distinctive natural blue color of the copper complex
- Focus on extracellular matrix remodeling and collagen synthesis
Product Specifications: Glow 70mg GHK-CU
| Specification | Details |
|---|---|
| Product Name | Glow GHK-Cu (Copper Tripeptide-1) |
| Chemical Name | Glycyl-L-Histidyl-L-Lysine Copper Complex |
| Peptide Sequence | Gly-His-Lys (Cu²⁺) |
| Molecular Formula | C₁₄H₂₃CuN₆O₄ |
| Molecular Weight | 401.91 g/mol |
| CAS Number | 89030-95-5 |
| PubChem CID | 73587 |
| Strength per Vial | 70mg GHK-Cu complex |
| Grade Classification | Research Use Only (RUO) / Cosmetic Research Grade |
| Form | Lyophilized peptide powder (distinctive blue color) |
| Purity Level | ≥98% (HPLC verified, COA provided per batch) |
| Intended Application | In vitro research & cosmetic formulation development only – NOT for human injection or therapeutic use |
| Storage Conditions | -20°C to -80°C (lyophilized form); 2-8°C after reconstitution |
| Appearance (Solution) | Blue-colored solution (characteristic of Cu²⁺ complex) |
Each batch undergoes comprehensive quality control including HPLC purity verification, mass spectrometry for molecular weight confirmation, copper content analysis, and sterility testing to ensure researchers receive pharmaceutical-grade materials for reliable experimental outcomes.
Understanding GHK-Cu: The Copper Peptide Complex
Discovery & Natural Occurrence
GHK-Cu was first isolated in 1973 by Dr. Loren Pickart from human plasma albumin. This naturally occurring tripeptide demonstrates a remarkably high affinity for copper(II) ions, forming a stable copper complex with a distinctive blue color and exceptional biological activity.
Natural Occurrence in Human Biology
- Plasma Concentration: ~200 ng/mL (10⁻⁷ M) at age 20
- Age-Related Decline: Drops to ~80 ng/mL by age 60
- Distribution: Found in plasma, saliva, and urine
- Endogenous Release: Released from tissue proteins (especially collagen and SPARC glycoprotein) following injury
- Biological Role: Functions as an emergency response molecule and copper carrier
The GHK Sequence in Nature
The GHK tripeptide sequence is naturally present in the alpha-2(I) chain of Type I collagen and can be liberated by proteases at wound sites. The glycoprotein SPARC (Secreted Protein Acidic and Rich in Cysteine), always present at tissue remodeling sites, releases GHK-Cu upon proteolytic breakdown.
Mechanism of Action: Multi-Pathway Modulation
GHK-Cu operates through several interconnected mechanisms, making it one of the most pleiotropic peptides in regenerative research:
1. Gene Expression Modulation
Landmark research using the Broad Institute’s Connectivity Map revealed that GHK influences gene expression on a massive scale:
- Scope: Modulates expression of 31.2% of human genes (at ≥50% change threshold)
- Direction: Upregulates 59% of affected genes, suppresses 41%
- Pathways: Affects genes involved in tissue repair, inflammation, oxidative stress, cell proliferation, and apoptosis
- Gene Categories: Resets gene expression patterns to resemble younger, healthier tissue
2. Copper Chelation & Delivery
The copper complex structure provides unique biological advantages:
- Stability Constant: Log₁₀ = 16.44 (extremely high affinity for Cu²⁺)
- Coordination Structure: Cu²⁺ coordinated by histidine imidazole nitrogen, glycine alpha-amino nitrogen, and deprotonated amide nitrogen
- Copper Delivery: Modulates cellular copper uptake and bioavailability
- Enzymatic Cofactor: Copper serves as cofactor for lysyl oxidase and lysyl hydroxylase (essential for collagen cross-linking)
3. Extracellular Matrix (ECM) Remodeling
- Collagen Synthesis: Increases Type I and Type III collagen production (up to 70% increase in laboratory studies)
- Elastin Production: Stimulates elastin synthesis for tissue flexibility
- Glycosaminoglycan (GAG) Synthesis: Enhances production of structural polysaccharides
- Decorin Stimulation: Increases decorin (proteoglycan regulating collagen synthesis and organization)
- Matrix Metalloproteinases (MMPs): Stimulates MMP-1 and MMP-2 for controlled matrix remodeling
- TIMPs: Increases Tissue Inhibitor of Metalloproteinases (TIMP-1) for balanced turnover
4. Growth Factor Stimulation
GHK-Cu promotes release of multiple growth factors critical for tissue regeneration:
- VEGF (Vascular Endothelial Growth Factor): Promotes angiogenesis and blood vessel formation
- BDNF (Brain-Derived Neurotrophic Factor): Supports neuronal survival and regeneration
- BMP-2 (Bone Morphogenetic Protein 2): Involved in bone and cartilage formation
- TGF-β Modulation: Influences transforming growth factor beta signaling
5. Antioxidant & Anti-Inflammatory Actions
- Free Radical Scavenging: Quenches reactive oxygen species (ROS) and lipid peroxidation products
- 4-HNE Neutralization: Quenches 4-hydroxy-trans-2-nonenal (toxic lipid peroxidation product)
- Acrolein Quenching: Neutralizes acrolein (age-related toxic aldehyde)
- Iron Regulation: Inhibits ferritin iron release in damaged tissues
- Cytokine Modulation: Reduces pro-inflammatory cytokines (IL-6, TNF-α)
6. Cellular Processes Enhanced
- Fibroblast Proliferation: Increases fibroblast activity and migration
- Keratinocyte Migration: Enhances re-epithelialization
- Endothelial Cell Growth: Supports blood vessel formation
- Immune Cell Recruitment: Attracts immune cells to injury sites
- Stem Cell Function: May help restore replicative vitality to aged cells
Research Context: In Vitro vs. In Vivo vs. Clinical
Important distinctions for researchers:
- In Vitro Data: Extensive cell culture studies (fibroblasts, keratinocytes, endothelial cells)
- Animal Models: Demonstrated wound healing, tissue repair in rats, rabbits, mice
- Human Topical Studies: Multiple small clinical trials showing cosmetic benefits (skin thickness, wrinkles, firmness)
- Injectable Data: Very limited human data; FDA has raised safety concerns for injectable forms
- Long-Term Safety: Decades of cosmetic use without major issues; injectable safety profile less established
Research Applications for GHK-Cu
In Vitro Research Models
Cell Culture Studies
- Fibroblast Investigations: Collagen synthesis, proliferation, gene expression studies
- Keratinocyte Research: Migration assays, differentiation pathways, barrier function
- Endothelial Cell Models: Angiogenesis assays, VEGF signaling, tube formation
- Stem Cell Research: Replicative capacity, senescence markers, differentiation potential
- Cancer Cell Lines: Apoptosis induction, growth inhibition mechanisms
Gene Expression Analysis
- Microarray studies examining global gene expression changes
- qPCR validation of specific gene targets
- Transcriptomic pathway analysis
- Epigenetic modification studies
- Comparison of young vs. aged cellular responses
Biochemical Assays
- Collagen synthesis quantification (ELISA, Western blot)
- MMP/TIMP activity measurement
- Oxidative stress markers (ROS, lipid peroxidation)
- Cytokine profiling (IL-6, TNF-α, TGF-β)
- Copper-binding kinetics and stability studies
Preclinical Animal Research
Published animal studies have examined GHK-Cu in multiple research models:
Wound Healing Models
- Dermal Wounds: Accelerated wound contraction, epithelialization, granulation tissue formation
- Diabetic Wounds: Improved healing in diabetic rat models with enhanced collagen synthesis
- Ischemic Wounds: Reduced healing time, decreased MMP-2/MMP-9, lower TNF-β levels
- Radiation-Damaged Tissue: Restored fibroblast viability post-radiation exposure
Skin Aging Models
- UV damage and photoaging studies
- Intrinsic aging biomarker analysis
- Collagen density measurements
- Elastic fiber organization
Neurological Research Models
- Neurodegenerative disease models (Alzheimer’s-related research)
- Copper dysregulation in brain tissue
- Amyloid-beta interactions
- Oxidative stress in neuronal tissue
Cosmetic Science Research
Formulation Development
- Stability Studies: pH effects, temperature cycling, light exposure, oxidation prevention
- Delivery Systems: Liposomal encapsulation, nano-lipid carriers, penetration enhancers
- Excipient Compatibility: Interaction with common cosmetic ingredients
- Skin Permeation: Stratum corneum penetration studies, bioavailability assessment
Efficacy Testing
- Clinical trial design for anti-aging claims
- Instrumental measurements (profilometry, corneometry, colorimetry)
- Histological analysis of skin biopsies
- Consumer perception studies
Analytical Method Development
- HPLC Methods: Purity determination, stability indicating assays
- Mass Spectrometry: Molecular weight confirmation, peptide sequencing
- Copper Content Analysis: ICP-MS, atomic absorption spectroscopy
- Bioassays: Cell-based potency assays, copper-binding capacity
Research Concentration Ranges
Published studies typically use:
- In Vitro: 1 pM to 100 nM (often most active at picomolar to nanomolar)
- Topical Formulations: 0.001-1% concentration in cosmetic studies
- Animal Models: Varies by application and delivery method
Note: These are research concentrations for laboratory studies only. Not recommendations for human use.
Bulk Supply & Wholesale Program for Research Institutions
The Glow 70mg GHK-CU formulation is available through our specialized bulk supply program designed for qualified research organizations, cosmetic manufacturers, and analytical laboratories.
Qualified Buyer Categories
- Academic Research Laboratories: University dermatology, biochemistry, and molecular biology departments
- Cosmetic Companies: Formulation R&D for topical skincare products
- Pharmaceutical Companies: Topical drug development and wound care research
- Contract Research Organizations (CROs): Cosmetic efficacy testing, safety studies
- Biotechnology Companies: Regenerative medicine and tissue engineering research
- Analytical Laboratories: Method development and quality control testing
- Raw Material Suppliers: Cosmetic ingredient distribution (with proper licensing)
Note: Compounding pharmacies seeking injectable formulations are not eligible due to FDA Category 2 restrictions (as of September 2023).
Flexible Quantity Tiers
- Starter Research Tier: 10-50 vials for pilot studies, formulation trials
- Cosmetic Development Tier: 51-200 vials for product development programs
- Large-Scale Production: 201-500 vials for commercial cosmetic manufacturing
- Enterprise/Distribution Tier: 500+ vials for ingredient suppliers and distributors
Quality Assurance Features
- INCI Compliant: Copper Tripeptide-1 designation for cosmetic formulations
- Batch Consistency: Validated synthesis protocols ensuring reproducible quality
- Certificate of Analysis: HPLC purity, MS verification, copper content, sterility data
- Cosmetic Grade Documentation: Suitable for use in cosmetic product development
- Stability Data: Long-term and accelerated stability studies provided
- Regulatory Support: Assistance with cosmetic ingredient declarations
Value-Added Services
- Technical consultation on formulation stability
- Custom packaging options for OEM customers
- Regulatory documentation support
- Rush processing for urgent research needs
- Long-term supply agreements with guaranteed pricing
GHK-Cu vs. Other Regenerative Peptides: Research Comparisons
| Parameter | GHK-Cu | BPC-157 | TB-500 |
|---|---|---|---|
| Peptide Length | 3 amino acids (tripeptide) | 15 amino acids | 7 amino acids (Tβ4 fragment) |
| Natural Source | Human plasma, collagen | Synthetic (gastric protein derivative) | Thymosin beta-4 fragment |
| Primary Mechanism | Gene expression modulation, copper delivery | VEGFR2 activation | Actin sequestration |
| Unique Feature | Copper chelation complex, affects 31% of human genes | Stable in gastric juice | Binds G-actin monomers |
| Research Applications | Skin regeneration, cosmetics, wound healing, gene studies | Tissue repair, angiogenesis, GI protection | Cell migration, wound healing, cytoskeleton |
| Cosmetic Use | ✅ Widely used (INCI: Copper Tripeptide-1) | ❌ Not approved for cosmetics | ❌ Not approved for cosmetics |
| FDA Status | Approved for topical cosmetics; Category 2 for injectables | Category 2 (compounding prohibited) | Category 2 (compounding prohibited) |
| WADA Status | ✅ Not prohibited | ❌ Banned (S0) | ❌ Banned (S0) |
| Clinical Data | Multiple human topical studies; decades of cosmetic use | Very limited (animal models primarily) | Limited (animal + small human studies) |
| Safety Profile | Well-established for topical use; injectable less studied | Primarily animal data | Limited human data |
Why GHK-Cu Stands Apart
- Regulatory Acceptance: Legal for cosmetic applications, decades of safe topical use
- Extensive Research Base: Published studies from 1973 to present (50+ years)
- Gene Expression Scope: Uniquely broad effects on human genome (31% of genes)
- Natural Occurrence: Endogenous human peptide with physiological role
- Commercial Availability: Widely available in skin care products globally
- Multi-Mechanism: Works through copper delivery, gene modulation, ECM remodeling, growth factors
Critical Distinction: Cosmetic vs. Injectable
Unlike BPC-157 and TB-500 (which face blanket restrictions), GHK-Cu has a bifurcated regulatory status:
- Topical/Cosmetic: Fully legal, widely used, extensive safety data
- Injectable: FDA Category 2 as of September 2023 due to limited injectable safety data and immunogenicity concerns
ALLUVI Position: We supply GHK-Cu exclusively for in vitro research and cosmetic formulation development. We do not support or encourage injectable human use.
Quality Documentation & Analytical Standards
Comprehensive Documentation Package
Each shipment of Glow 70mg GHK-CU includes:
Certificate of Analysis (COA)
- HPLC Purity Analysis: Chromatographic verification ≥98% purity
- Mass Spectrometry: Molecular weight confirmation (401.91 g/mol)
- Peptide Content: Quantitative assay of GHK peptide concentration
- Copper Content Analysis: Cu²⁺ quantification via ICP-MS or AAS
- Moisture Content: Karl Fischer analysis for lyophilized quality
- Microbial Testing: Sterility confirmation via USP methods
- Endotoxin Levels: LAL testing for biological research applications
- pH Analysis: Post-reconstitution pH verification
- Appearance: Confirmation of characteristic blue color in solution
Supporting Documentation
- Material Safety Data Sheet (MSDS/SDS): Complete safety, handling, disposal information
- Reconstitution Protocols: Detailed guidelines for proper solubilization
- Storage Guidelines: Temperature, light, and moisture protection recommendations
- Stability Data: Shelf-life and degradation studies under various conditions
- Amino Acid Sequence Verification: Confirming Gly-His-Lys sequence
- INCI Declaration: Copper Tripeptide-1 for cosmetic labeling
- Usage Guidelines: Concentration recommendations for cosmetic formulations
Quality Control Process
- Raw Material Verification: Amino acids and copper source authenticated
- Synthesis Monitoring: In-process testing at key stages
- Purification Validation: HPLC confirmation of purity post-purification
- Complex Formation: Spectroscopic verification of Cu²⁺ chelation
- Final Product Testing: Complete COA panel on finished product
- Stability Assessment: Real-time and accelerated stability protocols
- Release Testing: Final approval before shipment
Ordering Process for Qualified Researchers
Step 1: Initial Qualification Inquiry
Contact our research supply division at support@buyalluviretatrutide.co.uk with:
- Organization name and type (academic, cosmetic, pharmaceutical, etc.)
- Intended use (in vitro research, cosmetic formulation, analytical development)
- Principal investigator or R&D director credentials
- Estimated quantity requirements (initial and ongoing)
- Delivery location and timeline
Step 2: Application Review
Provide documentation as applicable:
- Research Institutions: University/institute affiliation, research protocols
- Cosmetic Companies: Business registration, cosmetic manufacturing license
- Ingredient Distributors: Distribution license, end-customer verification processes
- Contract Labs: Laboratory accreditation, client confidentiality agreements
- All Applicants: End-use declaration confirming research/cosmetic use only
Streamlined Process for Cosmetic Buyers
Cosmetic ingredient buyers benefit from simplified qualification due to GHK-Cu’s approved cosmetic status:
- Faster approval for established cosmetic manufacturers
- INCI documentation provided for product labeling
- Formulation support available
- Custom packaging for private label products
Step 3: Order Approval & Processing
Upon qualification approval:
- Receive volume-based pricing schedule
- Review minimum order quantities and payment terms
- Complete purchase order with legal attestations
- Select packaging options (standard or custom)
- Arrange secure payment and shipping logistics
- Receive order tracking and cold-chain monitoring information
Pricing & Cost Considerations
Pricing Structure
Factors affecting Glow 70mg GHK-CU pricing:
- Order Volume: Significant per-vial cost reductions at higher quantity tiers
- Purity Specifications: ≥98% purity copper complex commands premium pricing
- Documentation Package: Complete COA and regulatory support included
- Packaging Type: Standard or custom packaging options
- Delivery Requirements: Standard vs. expedited shipping, international logistics
- Standing Orders: Scheduled deliveries may receive preferential rates
Cost Comparison: GHK-Cu Value Proposition
When evaluating GHK-Cu suppliers, consider:
- Copper Complex Integrity: Proper Cu²⁺ chelation vs. free peptide
- Batch Consistency: Reproducible results reducing failed experiments
- Cosmetic-Grade vs. Research-Only: INCI compliance for commercial products
- Stability: Proper formulation preventing copper dissociation
- Technical Support: Formulation guidance, troubleshooting assistance
- Documentation: Complete regulatory support for product development
Typical Applications by Volume
| Application Type | Typical Order Size | Use Case |
|---|---|---|
| Academic Research | 10-50 vials | Cell culture studies, gene expression analysis |
| Formulation Development | 51-100 vials | Stability testing, efficacy trials, prototype batches |
| Cosmetic Production | 101-500 vials | Commercial product manufacturing |
| Ingredient Distribution | 500+ vials | Supply to cosmetic formulators, private label |
Request detailed pricing by contacting our wholesale team at support@buyalluviretatrutide.co.uk with your specific requirements.
Packaging, Storage & Logistics Excellence
Pharmaceutical-Grade Packaging
Primary Packaging
- Type I borosilicate glass vials (USP/Ph.Eur. compliant)
- Blue-tinted glass option (protects from light degradation)
- Lyophilized rubber stoppers meeting pharmaceutical standards
- Aluminum crimp seals with tamper-evident features
- Nitrogen backfilling to prevent oxidation
Secondary Packaging
- Moisture-barrier foil pouches with desiccants
- Light-protective packaging (copper complex is photosensitive)
- Impact-resistant cushioning for transit protection
- Temperature monitoring labels
- Clear labeling: “Copper Tripeptide-1 (GHK-Cu) – Research/Cosmetic Use Only”
Optimal Storage Conditions
| Storage Phase | Temperature | Duration | Additional Considerations |
|---|---|---|---|
| Lyophilized (Sealed) | -20°C to -80°C | 24-36 months | Protect from light and moisture; blue color stable |
| Refrigerated Storage | 2-8°C | 12-18 months | Acceptable for unopened vials if freezer unavailable |
| Reconstituted Solution | 2-8°C | 7-14 days max | Protect from light; monitor blue color (fading = degradation) |
| Working Formulations | Per formulation | Stability-dependent | pH control critical; antioxidants may help |
Stability Considerations
GHK-Cu stability is influenced by several factors:
- pH Sensitivity: Most stable at pH 5.5-7.0; copper dissociation at extreme pH
- Light Exposure: Blue color fades with photodegradation; store in dark
- Temperature: Accelerated degradation above 25°C
- Oxidation: Minimize air exposure; nitrogen atmosphere preferred
- Aqueous Solution: Time-dependent degradation; aliquot for single use
Cold-Chain Logistics
- Validated pharmaceutical cold-chain carriers
- Dry ice or gel pack configurations based on transit duration
- Real-time temperature data loggers with post-delivery reports
- Insurance coverage for high-value shipments
- Signature-required delivery to authorized personnel
International Shipping
- Export documentation and customs clearance support
- HS code classification for cosmetic ingredients
- Compliance verification with destination country regulations
- Multilingual documentation as required
- Special handling for EU cosmetic ingredient regulations
Regulatory Compliance & Legal Status
✅ GHK-Cu: Bifurcated Regulatory Status
GHK-Cu has distinctly different regulatory statuses depending on application:
APPROVED USES:
- Cosmetic Ingredient: Legal worldwide under INCI name “Copper Tripeptide-1”
- Topical Products: Creams, serums, lotions, masks – fully approved
- In Vitro Research: Laboratory studies, cell culture, analytical work
- Cosmetic R&D: Formulation development, efficacy testing
RESTRICTED USES:
- Injectable Forms: FDA Category 2 (September 2023) – compounding prohibited
- Therapeutic Claims: Not approved for treatment, cure, or prevention of disease
- Oral Supplements: Not approved as dietary supplement in US
FDA Regulatory Status (United States)
Cosmetic Use – APPROVED ✅
- INCI Name: Copper Tripeptide-1
- Status: Fully legal cosmetic ingredient
- Typical Concentrations: 0.001-1% in commercial products
- Labeling Requirements: INCI declaration on ingredient list
- Safety: Decades of safe topical use without major issues
Injectable Forms – RESTRICTED ⚠️
September 2023 FDA Action: Injectable GHK-Cu added to Category 2 bulk drug substances
- Rationale: “FDA has identified significant safety risks” including:
- Potential for immunogenicity (immune system reactions)
- Risk of peptide aggregation in injectable formulations
- Peptide-related impurities in compounded products
- Limited human safety data for injectable use
- Impact: Compounding pharmacies prohibited from preparing injectable GHK-Cu under 503A
- Exception: Some use under 503B outsourcing facilities with enhanced oversight
Critical Distinction
Topical GHK-Cu: Safe, legal, extensively used
Injectable GHK-Cu: Restricted by FDA due to limited safety data
This bifurcated status reflects decades of cosmetic safety vs. minimal injectable clinical data.
International Regulatory Status
European Union
- Cosmetic Regulation (EC) No 1223/2009: Legal cosmetic ingredient
- CosIng Database: Listed as Copper Tripeptide-1
- Safety Assessment: Required per EU cosmetic regulations
- Concentration Limits: Generally safe at typical cosmetic levels
Other Jurisdictions
- Canada: Permitted cosmetic ingredient; not approved as therapeutic
- Australia (TGA): Cosmetic use permitted; therapeutic use requires approval
- Japan: Approved cosmetic ingredient under MHLW regulations
- China: Permitted in cosmetics; specific registration may be required
WADA Status (Athletic Use)
✅ NOT PROHIBITED
- GHK-Cu is NOT on the WADA Prohibited List
- Athletes may use GHK-Cu topically without sanctions
- This contrasts sharply with BPC-157 and TB-500 (both banned S0)
Buyer Responsibilities
All purchasers must:
- Use GHK-Cu only for approved applications (research, cosmetic formulation)
- Comply with local cosmetic ingredient regulations
- NOT market or distribute for human injection without regulatory approval
- Provide proper INCI labeling in cosmetic products
- Maintain quality documentation for audits
- Sign end-use declarations confirming compliant use
ALLUVI Compliance Commitment
We maintain strict compliance by:
- Clear positioning as cosmetic/research ingredient
- Providing INCI documentation for cosmetic customers
- Refusing sales for injectable human use
- Transparent communication about regulatory status
- Complete documentation supporting legal use
- Regular legal review of compliance programs
Safety Profile & Research Considerations
Established Safety for Topical/Cosmetic Use
GHK-Cu has an extensive safety record for topical applications:
Positive Safety Indicators
- Decades of Use: Cosmetic ingredient since late 1980s
- Widespread Exposure: Thousands of cosmetic products worldwide
- Clinical Studies: Multiple double-blind trials showing good tolerability
- Low Adverse Event Rate: Minimal reported side effects in topical use
- Natural Occurrence: Endogenous peptide, not foreign to human biology
Reported Topical Side Effects (Rare)
- Mild skin irritation or redness (typically at high concentrations)
- Allergic reactions (very rare; may be due to formulation excipients)
- Temporary tingling sensation upon application
- Blue discoloration if product oxidizes (aesthetic issue, not safety concern)
Injectable Safety Concerns
FDA’s Category 2 classification for injectable GHK-Cu reflects specific concerns:
Immunogenicity Risk
- Peptide Aggregation: Injectable formulations may form aggregates triggering immune response
- Impurities: Synthesis by-products or degradation products could be immunogenic
- Repeated Exposure: Multiple injections may lead to antibody formation
- Limited Data: No large-scale human studies on injectable immune reactions
Copper-Related Considerations
- Copper Homeostasis: Systemic injection affects whole-body copper balance
- Wilson’s Disease: Contraindicated in copper metabolism disorders
- Zinc Interaction: Copper supplementation can deplete zinc
- Oxidative Stress: Free copper (if complex dissociates) can generate ROS
Anecdotal Injectable Reports
Unverified online reports from self-experimenters mention:
- Injection site reactions (pain, swelling, inflammation)
- Temporary fatigue or lethargy
- Headaches
- Skin changes (acne, rashes in some users)
- Blood pressure changes (unconfirmed)
Note: These are anecdotal, uncontrolled observations. Clinical data is lacking.
Theoretical Concerns for All Routes
Cancer Cell Interactions
- In vitro studies show GHK-Cu can induce apoptosis in some cancer cell lines (neuroblastoma, breast cancer)
- However, growth factor stimulation (VEGF, etc.) could theoretically support angiogenesis in existing tumors
- Current Evidence: Some anti-cancer effects in vitro, but insufficient human data
- Prudent Approach: Caution in individuals with known or suspected cancer
Copper Overload Syndromes
- Contraindicated in Wilson’s disease (genetic copper accumulation disorder)
- Use caution in individuals with liver disease (copper elimination impaired)
- Monitor copper and zinc levels with repeated or high-dose use
Research Best Practices
For laboratory investigations:
- Use appropriate controls (copper alone, peptide alone, vehicle)
- Monitor copper complex integrity (blue color, spectroscopy)
- Account for pH effects on stability and activity
- Verify concentration-response relationships (often active at picomolar levels)
- Consider time-dependent effects (gene expression changes take hours to days)
- Report negative or null findings to reduce publication bias
ALLUVI Position on Safety
We acknowledge:
- Topical GHK-Cu: Excellent safety profile, decades of use, minimal issues
- Injectable GHK-Cu: Limited safety data, legitimate FDA concerns, not recommended
- Research Applications: Appropriate for in vitro and cosmetic development with proper handling
We provide this product exclusively for research and cosmetic formulation. We do not encourage or support human injection.
Technical Support & Research Resources
Dedicated Technical Assistance
ALLUVI provides comprehensive support to Glow GHK-Cu customers:
Formulation Support (Cosmetic Applications)
- Concentration recommendations for different product types
- pH optimization for stability and activity
- Excipient compatibility guidance
- Stabilizer recommendations (antioxidants, chelators)
- Solubility optimization strategies
- Color stability preservation techniques
Research Methodology Consultation
- Reconstitution protocols for different applications
- Stock solution preparation and storage
- Dilution strategies for picomolar to micromolar ranges
- Cell culture application guidelines
- Gene expression study design recommendations
Analytical Method Support
- HPLC method parameters for GHK-Cu quantification
- Spectroscopic verification of copper complex
- Stability-indicating assay development
- Copper content determination methods
- Peptide sequencing confirmation
Troubleshooting Assistance
- Color fading/stability issues
- Precipitation or turbidity problems
- pH-related activity changes
- Inconsistent experimental results
- Storage degradation concerns
Scientific Literature Resources
Key peer-reviewed resources for researchers:
Foundational Research
- Original Discovery: Pickart L. (1973) – GHK isolation from human plasma
- Gene Expression: Pickart L., Margolina A. (2018) “Regenerative and Protective Actions of the GHK-Cu Peptide in the Light of the New Gene Data” – Int. J. Mol. Sci.
- Skin Regeneration: Multiple PMC articles on GHK as natural modulator of cellular pathways
Mechanism Studies
- Copper chelation dynamics and complex structure
- Matrix metalloproteinase regulation
- Collagen synthesis pathway activation
- Antioxidant and anti-inflammatory mechanisms
Clinical & Applied Research
- Cosmetic clinical trials (skin thickness, wrinkles, firmness)
- Wound healing studies in animal models
- Formulation stability research
- Skin penetration and bioavailability studies
Safety & Regulatory
- FDA Bulk Drug Substance Category determinations
- Cosmetic ingredient safety assessments
- Immunogenicity concerns for injectable forms
- Long-term topical use safety reviews
Frequently Asked Questions
Is GHK-Cu legal to purchase and use?
Yes, with important distinctions:
- Cosmetic/Topical Use: Fully legal worldwide as “Copper Tripeptide-1”
- In Vitro Research: Legal for laboratory studies
- Injectable Use: Restricted in US (FDA Category 2 as of Sept 2023)
- WADA Status: NOT prohibited for athletes (unlike BPC-157/TB-500)
Can I use GHK-Cu for personal anti-aging injections?
NO. ALLUVI does not sell GHK-Cu for injectable human use. While topical cosmetic use is safe and legal, injectable use:
- Is restricted by FDA due to safety concerns
- Lacks sufficient human clinical data
- Carries immunogenicity risks
- Should only be considered under physician supervision in jurisdictions where permitted
What purity level is Glow 70mg GHK-Cu?
Each batch is verified at ≥98% purity via HPLC, with comprehensive testing including:
- High-Performance Liquid Chromatography (HPLC) for purity
- Mass spectrometry confirmation of molecular weight (401.91 g/mol)
- Copper content analysis ensuring proper Cu²⁺ chelation
- Spectroscopic verification of complex integrity (blue color)
- Batch-specific Certificate of Analysis with all data
How does GHK-Cu compare to retinol for cosmetic applications?
GHK-Cu and retinol work through different mechanisms:
| Feature | GHK-Cu | Retinol |
|---|---|---|
| Mechanism | Gene modulation, copper delivery, ECM remodeling | Retinoic acid receptor activation |
| Irritation Potential | Low (generally well-tolerated) | Moderate to high (retinization period) |
| Photosensitivity | None (but photodegrades in light) | Yes (increases sun sensitivity) |
| Pregnancy Safety | Limited data; caution advised | Contraindicated (teratogenic) |
| Formulation Stability | Moderate (pH and light sensitive) | Poor (highly unstable) |
What is the shelf life of GHK-Cu?
- Lyophilized (sealed), -20°C: 24-36 months
- Refrigerated (unopened), 2-8°C: 12-18 months
- Reconstituted solution, 2-8°C: 7-14 days maximum
- In cosmetic formulations: Depends on formulation (stability testing required)
Why is my GHK-Cu solution blue?
The distinctive blue color is normal and indicates proper copper chelation. The color comes from Cu²⁺ coordination within the peptide complex. Color fading indicates degradation (copper dissociation or peptide breakdown) and loss of activity.
Can GHK-Cu be combined with other peptides in cosmetic formulations?
Yes, GHK-Cu is often combined with other peptides in commercial products:
- Matrixyl (Palmitoyl Pentapeptide): Complementary collagen stimulation
- Argireline (Acetyl Hexapeptide): Muscle relaxation + matrix remodeling
- Copper-Free GHK: Some formulators use both forms
Formulation compatibility testing is essential.
Who can purchase Glow 70mg GHK-Cu?
Qualified buyers include:
- Academic research institutions (universities, research centers)
- Cosmetic companies (skincare, haircare manufacturers)
- Pharmaceutical companies (topical drug development)
- Contract research organizations (CROs)
- Raw material distributors (with proper licensing)
- Analytical laboratories (method development, QC)
Does GHK-Cu need to be refrigerated during shipping?
Yes. We ship all GHK-Cu with cold-chain logistics:
- Dry ice or gel packs maintaining 2-8°C
- Temperature data loggers monitoring entire transit
- Insulated packaging rated for transit duration
- If temperature excursion occurs, we investigate and may reship
What concentration should I use in cosmetic formulations?
Typical cosmetic concentrations (based on published studies and commercial products):
- Facial Serums/Creams: 0.1-1% (higher concentrations common)
- Eye Creams: 0.05-0.5% (lower for sensitive eye area)
- Hair Care Products: 0.01-0.1%
- Research Studies: Often use higher concentrations for demonstrating efficacy
Conduct stability and safety testing for your specific formulation and concentration.
Why Choose ALLUVI for GHK-Cu Supply
Cosmetic-Grade Excellence
- INCI Compliant: Copper Tripeptide-1 documentation for cosmetic labeling
- Pharmaceutical Manufacturing: ISO-certified facilities with GMP-style processes
- Copper Complex Integrity: Spectroscopically verified Cu²⁺ chelation
- Consistent Blue Color: Visual confirmation of product quality
- Batch Reproducibility: Validated synthesis ensuring product consistency
Comprehensive Documentation
- Complete Certificate of Analysis (HPLC, MS, copper content, sterility)
- INCI declarations for cosmetic products
- Stability data supporting shelf-life claims
- Regulatory support for cosmetic compliance
- Formulation guidelines and concentration recommendations
Transparent Regulatory Positioning
- Clear Legal Status: Honest about topical vs. injectable distinctions
- FDA Awareness: Acknowledge Category 2 injectable restrictions
- No False Claims: We don’t market for prohibited uses
- Educational Approach: Provide complete safety and regulatory information
- Ethical Sales: Refuse orders for non-compliant applications
Technical Expertise
- PhD-level scientists available for consultation
- Cosmetic formulation guidance
- Stability optimization recommendations
- Analytical method development support
- Literature review assistance
Customer Service Excellence
- Responsive support team (email, phone, video consultations)
- Custom packaging for private label customers
- Flexible ordering to accommodate R&D cycles
- Long-term supply agreements for commercial manufacturers
- Rush processing available for urgent needs
Competitive Advantages
| Feature | ALLUVI Standard |
|---|---|
| Purity Guarantee | ≥98% HPLC verified with copper complex integrity |
| Color Verification | Characteristic blue color confirms proper chelation |
| Regulatory Expertise | INCI documentation, cosmetic compliance support |
| Cold-Chain Integrity | Temperature-controlled logistics with monitoring |
| Technical Support | Formulation guidance from peptide chemistry experts |
| Ethical Operations | Transparent about legal uses, refuse non-compliant orders |
Get Started with Glow 70mg GHK-Cu
Whether you’re conducting academic research on gene expression, developing the next breakthrough skincare product, or formulating professional cosmetic lines, ALLUVI provides the high-quality GHK-Cu and technical support your work demands.
Next Steps for Qualified Buyers
- Review Intended Use: Confirm your application is compliant (research, cosmetic formulation)
- Prepare Documentation: Gather institutional credentials or business registration
- Contact Our Team: Email support@buyalluviretatrutide.co.uk with your requirements
- Complete Qualification: Submit required documentation
- Receive Pricing: Review volume-based pricing and terms
- Place Order: Complete purchase agreement
- Receive Shipment: Cold-chain delivery with comprehensive documentation
- Access Support: Utilize formulation and technical consultation
Ready to Order Glow GHK-Cu?
Contact our cosmetic & research supply team to:
- Discuss your specific application (cosmetic, research, analytical)
- Request detailed pricing for your quantity needs
- Receive INCI documentation and formulation guidance
- Establish long-term supply agreements for manufacturing
- Schedule technical consultation with our peptide experts
Important Disclaimers & Legal Notices
Research & Cosmetic Use Only – Comprehensive Legal Disclaimer
Glow 70mg GHK-Cu is manufactured and sold exclusively for in vitro research and cosmetic formulation purposes.
- Cosmetic Ingredient: Legal for topical cosmetic use as “Copper Tripeptide-1” per INCI standards.
- Research Use: Intended for laboratory investigations, cell culture studies, and analytical method development.
- NOT for Injectable Human Use: Injectable forms restricted by FDA (Category 2, September 2023) due to immunogenicity and safety concerns.
- Not FDA-Approved Drug: GHK-Cu is not FDA-approved for treatment, cure, or prevention of any disease.
- No Medical Claims: No claims are made or implied regarding therapeutic, diagnostic, or health benefits.
- Qualified Buyers Only: Sales restricted to verified research institutions, cosmetic manufacturers, and licensed distributors.
- Buyer Responsibility: Purchasers assume full responsibility for compliance with all applicable laws and regulations.
- Proper Use Attestation: By purchasing, you confirm use only for approved applications (research, cosmetic formulation).
- No Warranty for Misuse: Sold “as is” for stated purposes. No warranty for fitness outside research/cosmetic use.
- Limitation of Liability: ALLUVI shall not be liable for misuse, unauthorized use, or consequences from non-compliant applications.
SAFETY NOTICE: While GHK-Cu has decades of safe topical/cosmetic use, injectable forms carry potential risks including immune reactions, copper imbalance, and unknown long-term effects. Topical cosmetic use is well-established and safe; injectable use is restricted and should only occur under qualified medical supervision in jurisdictions where permitted.
By purchasing this product, you acknowledge that you have read, understood, and agreed to comply with all terms, restrictions, and legal obligations outlined in this document and in the purchase agreement.





Frederick Edward –
I’ve been taking Glow 70mg for a few weeks, and the transformation in my daily rhythm has been remarkable. My energy no longer dips in the afternoons, and I feel a sustained vitality that carries me through long workdays. It’s like my body finally found its balance.
Benjamin Edward –
Glow 70mg has helped me stay consistent with my fitness routine. I notice I recover faster after workouts and have more stamina to push through my sessions. It’s been a great boost for my lifestyle.
Isabella George –
I began taking Glow 70mg when my afternoons seemed to be a never-ending slump. After a week, I found that I could go through meetings without feeling too exhausted. I didn’t collapse on the couch when I got home since I still had enough stamina to make dinner and enjoy the evening.
Amelia Rose –
What surprised me most about Glow 70mg is the emotional steadiness it brings. I’m calmer, less reactive, and more patient in stressful situations. That inner equilibrium has made my relationships smoother and my days far more enjoyable.
Leo Siegfried –
Glow 70mg has given me a newfound sense of clarity. Tasks that used to feel overwhelming now seem manageable, and I approach my day with sharper focus and steadier motivation. It’s as if the fog has lifted, and I can finally operate at my full potential.